structure of i3 negative|Lewis Structure for I3 : Pilipinas n this lesson, we follow five systematic steps to draw the correct Lewis structure of I3-. Screen capture by Screencast-O-Matic (http://www.screencast-o-mati.
Hawaii Standard Time. Honolulu. Tue, 1:13:13 pm. Hawaii observes Hawaii Standard Time all year. There are no Daylight Saving Time clock changes.
PH0 · Lewis Structure of I3
PH1 · Lewis Structure for I3
PH2 · I3
PH3 · Hybridization of I3(
PH4 · How to Draw the Lewis Dot Structure for I3
We would like to show you a description here but the site won’t allow us.Send or request money, pay or split the bill in as easy as 1-2 tap with BDO Pay!
structure of i3 negative*******It is important to know the Lewis structure of a molecule to understand its physical properties, hybridization, and shape of the molecule. .Hybridization of I3(-) is sp3d - Understand the molecular geometry and Hybridization of I3(-). Learn to determine the shape and hybridization of iodine .
The triiodide ion (I3⁻) consists of a linear arrangement of three iodine (I) atoms, with the central I atom bonded to two terminal I atoms. It has 7 valence electrons per I atom, plus .Lewis Structure for I3I 3- has a negative charge (and is therefore a negative ion or anion). That means that it has an extra electron that needs to be taken into account. Since iodine (I) is below Period Three on . A step-by-step explanation of how to draw the I3 - Lewis Dot Structure (Triiodide ion). For the I3 - structure use the periodic table to find the total number of valence electrons for t.n this lesson, we follow five systematic steps to draw the correct Lewis structure of I3-. Screen capture by Screencast-O-Matic (http://www.screencast-o-mati.
This Lewis structure shows us that the triiodide ion has 22 valence electrons, a linear shape, and a negative charge. FAQs. What is the Lewis structure of I3-? The Lewis structure of I3- .

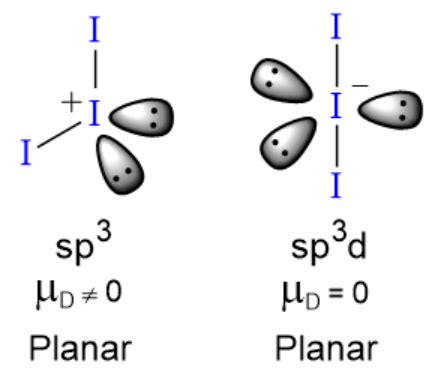
For the Lewis structure for I3- you have to take formal charges into account to find the best Lewis structure for the molecule. You should put brackets with an negative sign around the I3- . I3- (triiodide) lewis structure has three Iodine atoms (I). There are 2 single bonds between each Iodine atom (I). There are 3 lone pairs on all the three Iodine atom.Seven minus six minus two gives us a negative one charge. So all of this makes sense. We have the negative one charge here. That makes sense: we have a negative three so we can draw this with brackets so everyone can see that it is an ion. Put a negative up there. That's the Lewis structure for I3-. This is Dr. B, and thanks for watching. The triiodide ion (I3⁻) consists of a linear arrangement of three iodine (I) atoms, with the central I atom bonded to two terminal I atoms. It has 7 valence electrons per I atom, plus one additional electron due to the negative charge, totaling 22 electrons. The Lewis structure shows two single I-I bonds and three lone pairs on the central I . Hybridization of I3. When you draw the structure of any molecule, it is the 2d structure, but the hybridization of atoms and molecules can reveal the 3d representation of molecular shape. The hybridization of i3 is sp3d means three orbital take part in forming an i3 molecule viz one s, three p, and one d molecular orbital. In the periodic table, iodine lies in group 17. Hence, iodine has seven valence electrons.. Since I 3 – has three iodine atoms, so.. Valence electrons of three iodine atoms = 7 × 3 = 21. Now the I 3 – has a negative (-1) charge, so we have to add one more electron.. So the total valence electrons = 21 + 1 = 22. Second, find the total electron pairs; We have a total of .Drawing the Lewis Structure for I 3-Video: Drawing the Lewis Structure for I 3-For the I3- Lewis structure we first count the valence electrons for the I3- molecule using the periodic table. Once we know how many valence electrons there are in I3- we can distribute them around the central atom and attempt to fill the outer shells of each atom.
I2 + I- —-> I3- This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings. One of the major uses of this ion is due to its non-reactive property with starch which results in an identifiable blue-black color used a lot for identification. It is a major component of a lot of salts. Now, let us . Total valence electrons in I3- ion = valence electrons given by 3 iodine atoms + 1 more electron is added due to 1 negative charge = 7(3) + 1 = 22. Step 2: Select the central atom . In the above lewis dot structure of I3- ion, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will get the following .
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright . The Lewis structure of triiodide [I 3] – consists of three identical iodine (I) atoms. One I atom acts as the central atom while the other two iodine atoms act as outer atoms. There are a total of 5 electron density regions around the central I atom in the I 3 – Lewis structure. Out of the 5 electron density regions, there are 2 bond pairs and 3 lone pairs of electrons on the .70 More Lewis Dot Structures. I does not follow the octet rule. It will hold more than 8 electrons. Iodine having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons.
n this lesson, we follow five systematic steps to draw the correct Lewis structure of I3-. Screen capture by Screencast-O-Matic (http://www.screencast-o-mati.structure of i3 negativeFor the I3- Lewis structure we first count the valence electrons for the I3- molecule using the periodic table. Once we know how many valence electrons there are in I3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. . You should put brackets with an negative sign around the I3- Lewis .
I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle. A step-by-step explanation of how to draw the I3 - Lewis Dot Structure (Triiodide Ion).For the I3 - structure use the periodic table to find the total number.In chemistry, triiodide typically refers to the triiodide particle, I3. This anion, one of all the polyhalogen ions, consists of 3 iodine atoms. It’s fashioned by combining liquid solutions of halide salts and Iodine. Some salts of the ion are isolated, together with thallium(I) triiodide (Tl+[I3] −) and ammonia triiodide ([NH4]+[I3] − . The (ab initio) effective-potential theory developed by Ewig et al. is applied to the structures of the polyiodide ions, I 3 − and I 5 −.The bare ions I 3 − and I 5 − are found by optimization of the geometry to be symmetric and linear. The counterion environment, however, greatly influences the equilibrium structure.
This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and form. If you draw structures with extra bonds, they have to be correct. For example, a structure for phosphate that has two double bonds is not acceptable. Also, a structure for nitrate ion (NO 3 –) that has two double bonds is not acceptable, even though it gives you more zeroes. The structure with one double bond is the only acceptable one.
On this channel I share fashion styling tips, product reviews, and inspiring lifestyle content to help you enhance your everyday life! Subscribe and become a fellow “SEAChel" 🐚 and let’s .
structure of i3 negative|Lewis Structure for I3